Which of the Following Best Describes the Kinetic Theory
Atoms and molecules are always moving or vibrating. It explains how properties of gases are related to the attractive forces between particles.

Kinetic Theory Kinetic Theory Molecules Kinetic
The total kinetic energy of the two particles stays the same.
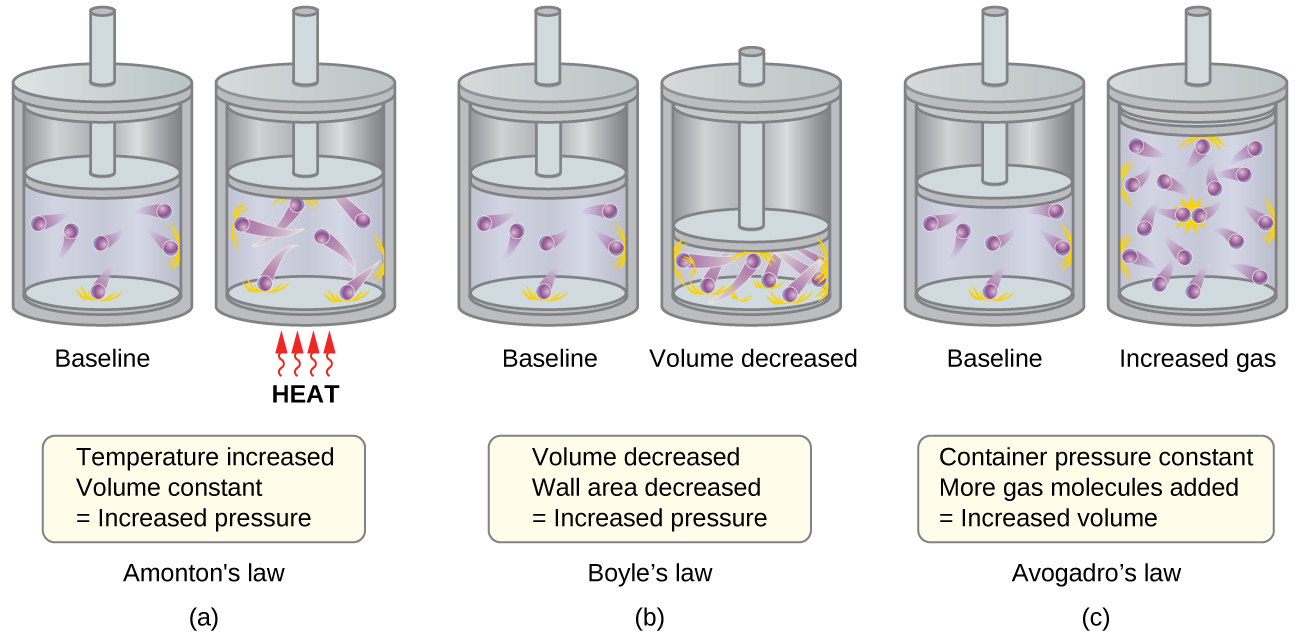
. Which of the following describes the kinetic molecular theory. Kinetics has to do with some kind of movement but also energy. D Gas particles are in constant motion at.
Energy is conserved when converted to another type of energy. Atoms rearrange in molecules to form new compounds. View the full answer.
The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only. What best describes the kinetic energy of two gas particles before and after a collision. The reason behind this is.
Which of the following correctly describes the movement of gas particles according to the kinetic theory of gases. Where is the pressure of the gas is the volume taken up by the gas is the temperature of. The kinetic theory of matter tells us a lot about different phases of matter.
Kinetic Molecular Theory KMT describes the experimentally discovered behavior of particles. According to the kinetic molecular theory of gas. 2 The space occupied by the molecules of gas in a container is very negligible.
The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. According to the statement which answer best describes how a substance would be affected if its temperature increased. The kinetic molecular theory can be used to explain the results Graham obtained when he studied the diffusion and effusion of gases.
KMT is most often referenced in relation to the behavior of. The key to this explanation is the last postulate of the kinetic theory which assumes that the temperature of a system is proportional to the average kinetic energy of its particles and nothing else. D The gas molecules are at low temperatures.
The kinetic molecular theory describes the properties of molecules in terms of motion kinetic energy and of temperature. The kinetic molecular theory assumes that the particles of an ideal gas 1 are in random constant t i ht-line motion 2 are arranged in a regular geometric pattern 3 have strong attractive forces between them 4 have collisions that result in the system losing energy. Which of the following best describes kinetic molecular theory definition of an ideal gas.
The statement that best describes the kinetic theory of matter is Matter is made up of particles that are in constant motion and have energy. Which of the following is the most energetic state of matter. Which of these describes a situation where gases are ideal according to the Kinetic Molecular Theory of gases.
The statement that best describes the kinetic theory of matter is Matter is made up of particles that are in constant motion and have energy. Which of the following best describes the kinetic theory. This theory states that all matter is made of small particles that are in random motion and that have space between them.
It describes the behavior of gases. The kinetic molecular theory describes the behavior of gasses in terms of particles in. As applied to gases the kinetic molecular theory has the following postulates.
Kinetic Molecular Theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. Unit 8 exercise 1 - ANSWER KEY. 1 When molecules collide with each other no energy is gained or lost.
3 These molecules always have linear motion. C The average speed of gas particles increases with decreasing temperature. The simplicity of this relationship is a big reason why we typically treat gases as ideal unless there is a good reason to do otherwise.
The three main components of the kinetic theory of gases are. Vinegar contains acetic acid CH 3 COOH which gives vinegar its sour taste and pungent smell. The Kinetic Molecular Theory and Grahams Laws.
What is the percent composition of carbon in acetic acid. A scientist examines a large pot of boiling water and a small cup of boiling water. Kinetics has to do with some kind of movement but also energy.
Kinetic Molecular Theory can be used to explain both Charles and Boyles Laws. Atoms and molecules are always moving or vibrating. 100 1 rating The answer to your question is explained below.
A All gas particles move with the same speed at a given temperature. B All gas particles move in curved lines in random directions. The substances average kinetic energy would increase.
The pressure volume and temperature of an ideal gas are related by a simple formula called the ideal gas law. The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion colliding elastically with one another and the walls of their container with average velocities determined by their absolute temperatures. C The gas molecules are at high pressures.
Chemistry Gases Kinetic Theory of Gases. B The gas molecules are close and sticky. It describes the behavior of condensed states.
A The gas molecules are far apart and moving quickly. Electron pairs move apart to form more stable molecules. According to Kinetic Theory of Gasesthe intermolecular forces between.
The theory is most often applied to gases but is helpful in explaining molecular behavior in all states of matter. The correct answer is matter is made up of particles that are in constant motion and have energy. Add up the weight of the entire molecule and divide by the weight of carbon answer choices.
Option-B is the correct answer in my opinion. It explains how macroscopic properties are related to the behavior of atoms. Which of the following best describes the kinetic theory.
No comments for "Which of the Following Best Describes the Kinetic Theory"
Post a Comment